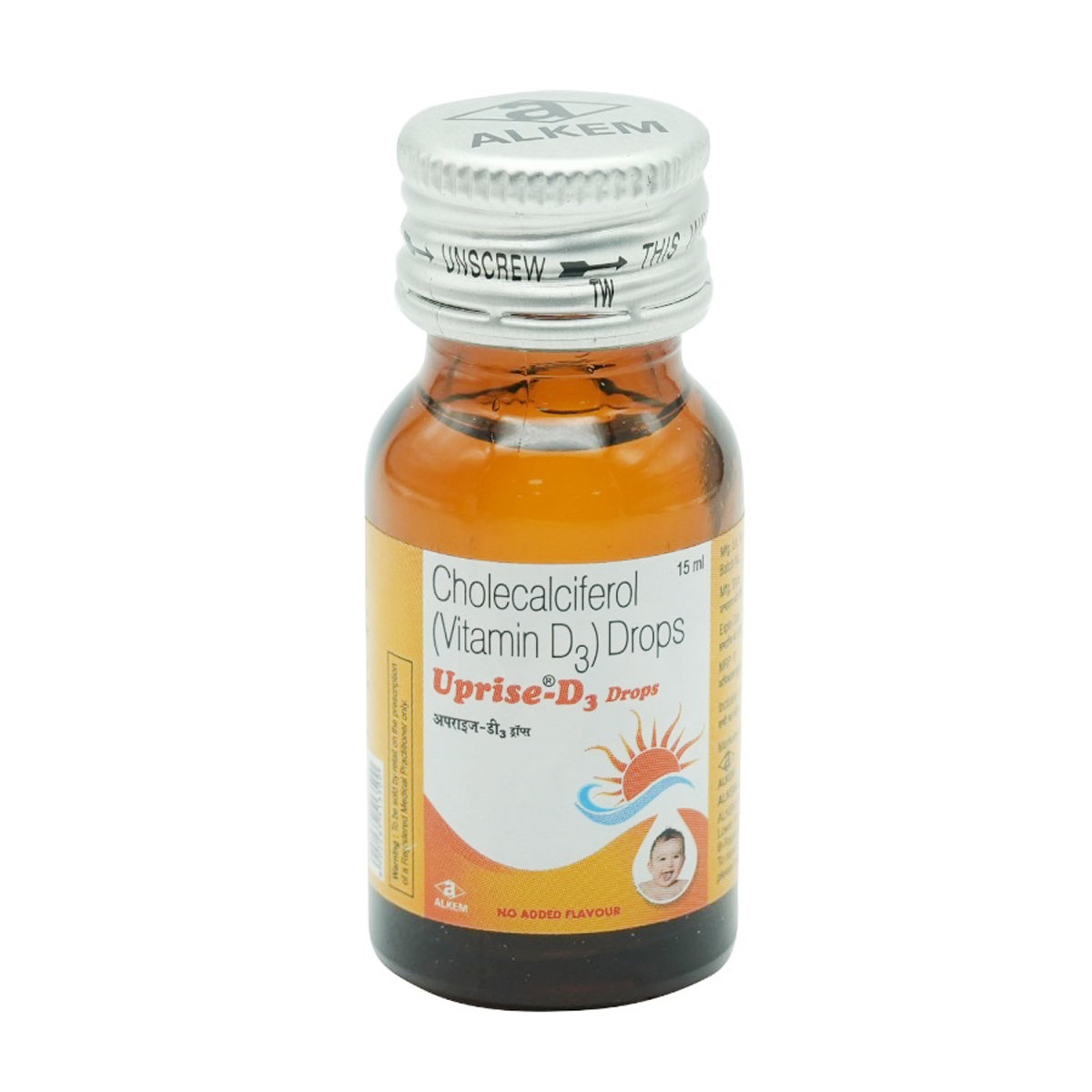
Uprise D3 Drops 15 ml
MRP ₹110.5
(Inclusive of all Taxes)
₹16.6 Cashback (15%)
About Uprise D3 Drops
Uprise D3 Drops belongs to the class of 'Vitamins', primarily used to treat low blood calcium levels. Uprise D3 Drops effectively treats various conditions in the body like Vitamin D deficiency, osteoporosis (weak and brittle bones), hypoparathyroidism (parathyroid glands make low levels of calcium in the body), latent tetany (a muscle disease with low blood calcium levels) and rickets or osteomalacia (softening or deforming of bones due to lack of calcium). Vitamin D deficiency occurs when your body has low Vitamin D levels and is caused due to inadequate nutrition, intestinal malabsorption or lack of sunlight exposure.
Uprise D3 Drops contains 'Cholecalciferol' a form of vitamin-D. It acts by promoting the absorption of calcium, phosphates and Vitamin A from different organs and helps in maintaining overall health.
Take Uprise D3 Drops as advised. Your physician will decide the dosage based on your medical condition. Uprise D3 Drops is likely safe to consume. In some cases, it may cause side effects like constipation, increased blood calcium levels, increased calcium levels in urine, vomiting, nausea. These side effects do not require medical attention and gradually resolve over time. If these side effects persist, please consult your physician immediately.
Tell your physician if you are allergic to Uprise D3 Drops. Chewable or dissolving tablets may contain sugar or aspartame, hence caution should be taken in diabetes and phenylketonuria (increased levels of an amino acid called phenylalanine). Pregnant or breastfeeding women should consult their physician before taking Uprise D3 Drops. Higher doses of Vitamin D than the recommended daily dose should be used in pregnant women only when advised by the doctor. Uprise D3 Drops passes into the breast milk, hence breastfeeding mothers need to seek medical advice before starting Uprise D3 Drops. Uprise D3 Drops is safe to use in children when recommended by the pediatrician. Uprise D3 Drops should be used with caution in hypercalcemia, renal impairment, heart diseases, kidney stones and hypervitaminosis D (having too much vitamin D).
Country of origin
Manufacturer/Marketer address
Online payment accepted

secured payment

india's most trusted pharmacy

genuine products
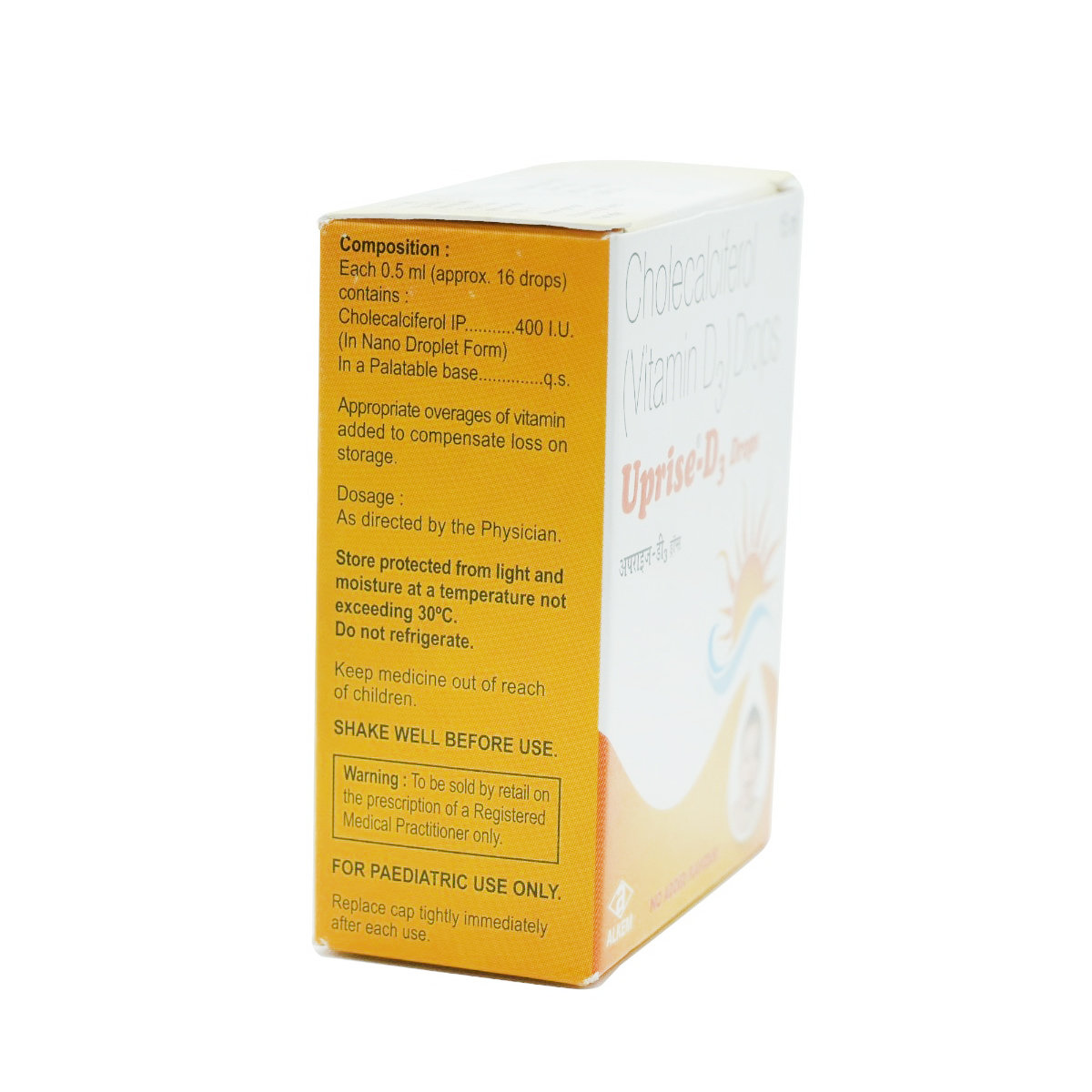
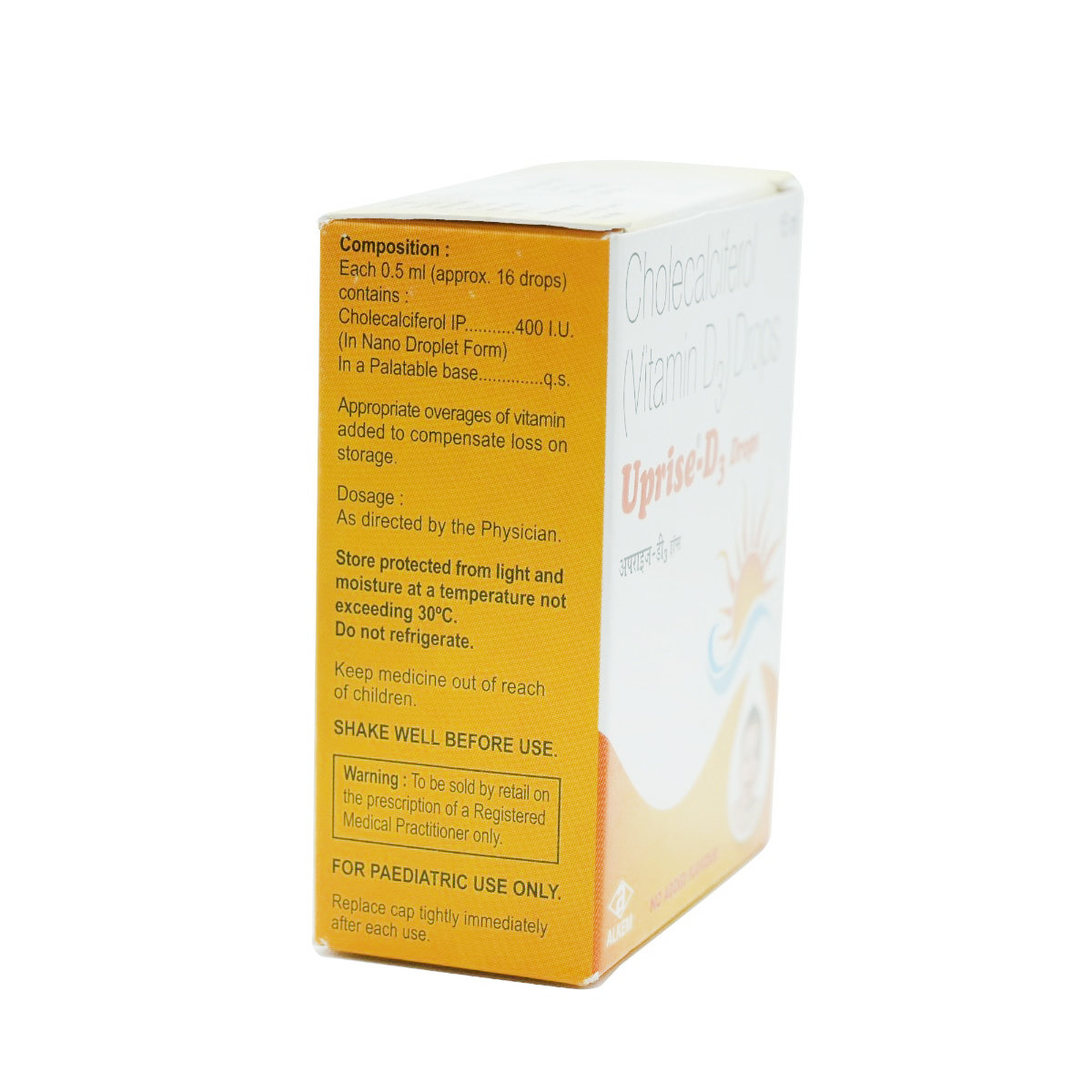
Composition :
Manufacturer/Marketer :
Consume Type :
Expires on or after :
Return Policy :
Provide Delivery Location
About Uprise D3 Drops
Uprise D3 Drops belongs to the class of 'Vitamins', primarily used to treat low blood calcium levels. Uprise D3 Drops effectively treats various conditions in the body like Vitamin D deficiency, osteoporosis (weak and brittle bones), hypoparathyroidism (parathyroid glands make low levels of calcium in the body), latent tetany (a muscle disease with low blood calcium levels) and rickets or osteomalacia (softening or deforming of bones due to lack of calcium). Vitamin D deficiency occurs when your body has low Vitamin D levels and is caused due to inadequate nutrition, intestinal malabsorption or lack of sunlight exposure.
Uprise D3 Drops contains 'Cholecalciferol' a form of vitamin-D. It acts by promoting the absorption of calcium, phosphates and Vitamin A from different organs and helps in maintaining overall health.
Take Uprise D3 Drops as advised. Your physician will decide the dosage based on your medical condition. Uprise D3 Drops is likely safe to consume. In some cases, it may cause side effects like constipation, increased blood calcium levels, increased calcium levels in urine, vomiting, nausea. These side effects do not require medical attention and gradually resolve over time. If these side effects persist, please consult your physician immediately.
Tell your physician if you are allergic to Uprise D3 Drops. Chewable or dissolving tablets may contain sugar or aspartame, hence caution should be taken in diabetes and phenylketonuria (increased levels of an amino acid called phenylalanine). Pregnant or breastfeeding women should consult their physician before taking Uprise D3 Drops. Higher doses of Vitamin D than the recommended daily dose should be used in pregnant women only when advised by the doctor. Uprise D3 Drops passes into the breast milk, hence breastfeeding mothers need to seek medical advice before starting Uprise D3 Drops. Uprise D3 Drops is safe to use in children when recommended by the pediatrician. Uprise D3 Drops should be used with caution in hypercalcemia, renal impairment, heart diseases, kidney stones and hypervitaminosis D (having too much vitamin D).
Uses of Uprise D3 Drops
Key Benefits
Uprise D3 Drops is used to treat low blood calcium levels. It effectively treats various conditions in the body like Vitamin D deficiency, osteoporosis, hypoparathyroidism, latent tetany and rickets or osteomalacia. Uprise D3 Drops contains Cholecalciferol (Vitamin D3). Cholecalciferol is a steroid hormone produced in the skin when exposed to ultraviolet light or obtained from food sources. It is a provitamin that is converted into vitamin after intake. It helps maintain blood calcium and phosphorus levels and mineralization of bone. Uprise D3 Drops is also used in the treatment of familial hypophosphatemia (a group of rare inherited disorders characterized by impaired kidney conservation of phosphate and in some cases, altered vitamin D metabolism).
Directions for Use
Storage
Side Effects of Uprise D3 Drops
- Constipation
- Increased blood calcium levels
- Increased calcium levels in urine
- Vomiting
- Nausea
- Chest pain, feeling short of breath
Drug Warnings
Tell your physician if you are allergic to Uprise D3 Drops. Chewable or dissolving tablets may contain sugar or aspartame, hence caution should be taken in diabetes and phenylketonuria (increased levels of an amino acid called phenylalanine). Pregnant or breastfeeding women should consult their physician before taking Uprise D3 Drops. Higher doses of Vitamin D than the recommended daily dose should be used in pregnant women only when advised by the doctor. Uprise D3 Drops passes into the breast milk, hence breastfeeding mothers need to seek medical advice before starting Uprise D3 Drops. Uprise D3 Drops is safe to use in children when advised by the doctor. Uprise D3 Drops should be used with caution in hypercalcemia, hyperparathyroidism, renal impairment, electrolyte imbalance, heart diseases, kidney stones and hypervitaminosis D (having too much vitamin D).
Drug Interactions
Drug-Drug Interactions: Uprise D3 Drops may interact with drugs treating high cholesterol levels (cholestyramine), anti-epileptic (carbamazepine, phenobarbital), antibiotics (doxycycline, neomycin, and chloramphenicol), drugs treating bone loss (alendronate), thyroid hormone (levothyroxine), diuretics (hydrochlorothiazide) and heart-related medicines (digoxin).
Drug-Food Interactions: Avoid or reduce the intake of caffeine, soft drinks and alcohol that inhibit calcium absorption.
Drug-Disease Interactions: Uprise D3 Drops is contraindicated in hypercalcemia, hyperparathyroidism, hypervitaminosis D, malabsorption syndrome, Vitamin D toxicity, heart/kidney/liver/blood vessel diseases, kidney stones, diabetes and phenylketonuria.
Drug-Drug Interactions Checker List
- CHOLESTYRAMINE
- CARBAMAZEPINE
- PHENOBARBITAL
- DOXYCYCLINE
- NEOMYCIN
- CHLORAMPHENICOL
- ALENDRONATE SODIUM
- LEVOTHYROXINE
- HYDROCHLOROTHIAZIDE
- DIGOXIN
Habit Forming
Diet & Lifestyle Advise
- Include dairy products like milk, yogurt, cheese or milk-based custard in your diet.
- Eat daily a serving of broccoli, cabbage, spinach and other green leafy vegetables.
- Include the best dietary sources of vitamin D, such as fish liver oils and vitamin D–fortified milk.
- Snack on calcium-rich nuts like Brazil nuts or almonds.
- Sprinkle sesame seeds over your food, vegetables and salads. Sesame seeds are high in calcium.
- Avoid or reduce the intake of caffeine, soft drinks and alcohol that inhibit calcium absorption.
- Replace the meat with tofu or tempeh for extra calcium in your food.
Special Advise
- Uprise D3 Drops may interfere with cholesterol tests, hence please inform your physician and laboratory staff that you are taking Uprise D3 Drops before undergoing blood tests.
- Clinical monitoring of serum electrolyte concentrations and cardiac function is recommended.
Disease/Condition Glossary
Vitamin D deficiency: When a person cannot get enough Vitamin D through food and exposure to sunlight, it leads to vitamin D deficiency. It often leads to thin, brittle, or misshapen bones.
Osteoporosis: It is a bone disease that weakens and brittle bones by decreasing bone density.
Tetany: A disease condition due to low levels of calcium (hypocalcemia) in the body that causes cramps and spasms in the hands, feet, and larynx (voice box).
Osteomalacia/Rickets: A disease caused by softening and weakening bones in children due to inadequate vitamin D.
Hypoparathyroidism: It is a disease characterized by low parathyroid hormone levels. This can cause low calcium levels and trigger tetany (muscle cramps, spasms, or tremors).
FAQs
Disclaimer
Alcohol
Safe if prescribed
Drinking alcohol can affect calcium absorption, hence it is advised to limit the alcohol intake while using Uprise D3 Drops.
Pregnancy
Consult your doctor
During pregnancy, use higher doses of Uprise D3 Drops than the daily dietary allowance only when advised by the doctor. Your physician will weigh the potential risks and benefits before recommending Uprise D3 Drops.
Breast Feeding
Consult your doctor
Consult your physician before taking Uprise D3 Drops if you are breastfeeding. Uprise D3 Drops can pass into the breast milk. If Uprise D3 Drops is used during breastfeeding, please monitor the mother and the infant's serum calcium levels.
Driving
Safe if prescribed
If you experience any dizziness while using Uprise D3 Drops, do not drive or operate machinery. Please consult your doctor if you experience any dizziness.
Liver
Consult your doctor
Let your physician know if you have any history of liver diseases before taking Uprise D3 Drops. Hepatic impairment/liver disease can alter the metabolic and therapeutic activity of certain Vitamin D forms.
Kidney
Consult your doctor
It is advised to seek physician advice before starting Uprise D3 Drops if you have kidney diseases like kidney stones or undergoing dialysis. Caution should be taken in patients undergoing dialysis to maintain adequate phosphorus levels and avoid ectopic calcification (calcium deposition).
Children
Safe if prescribed
The physician will suggest the dosage of Uprise D3 Drops based on the child's age and weight.
Uses of Uprise D3 Drops
Key Benefits
Uprise D3 Drops is used to treat low blood calcium levels. It effectively treats various conditions in the body like Vitamin D deficiency, osteoporosis, hypoparathyroidism, latent tetany and rickets or osteomalacia. Uprise D3 Drops contains Cholecalciferol (Vitamin D3). Cholecalciferol is a steroid hormone produced in the skin when exposed to ultraviolet light or obtained from food sources. It is a provitamin that is converted into vitamin after intake. It helps maintain blood calcium and phosphorus levels and mineralization of bone. Uprise D3 Drops is also used in the treatment of familial hypophosphatemia (a group of rare inherited disorders characterized by impaired kidney conservation of phosphate and in some cases, altered vitamin D metabolism).
Directions for Use
Storage
Drug Warnings
Tell your physician if you are allergic to Uprise D3 Drops. Chewable or dissolving tablets may contain sugar or aspartame, hence caution should be taken in diabetes and phenylketonuria (increased levels of an amino acid called phenylalanine). Pregnant or breastfeeding women should consult their physician before taking Uprise D3 Drops. Higher doses of Vitamin D than the recommended daily dose should be used in pregnant women only when advised by the doctor. Uprise D3 Drops passes into the breast milk, hence breastfeeding mothers need to seek medical advice before starting Uprise D3 Drops. Uprise D3 Drops is safe to use in children when advised by the doctor. Uprise D3 Drops should be used with caution in hypercalcemia, hyperparathyroidism, renal impairment, electrolyte imbalance, heart diseases, kidney stones and hypervitaminosis D (having too much vitamin D).
Therapeutic Class
Drug-Drug Interactions
Drug-Drug Interactions
Login/Sign Up
The combined use of aluminum hydroxide with Uprise D3 Drops 15 ml may increase the risk of toxicity.
How to manage the interaction:
Co-administration of Uprise D3 Drops 15 ml with Aluminium hydroxide can possibly result in an interaction, but it can be taken if your doctor has advised it. If you're having any of these symptoms like bone pain, muscle weakness, anemia, seizures, or dementia, it's important to contact your doctor right away. Do not stop using any medications without a doctor's advice.
The combined use of calcifediol with cholecalciferol can increase the risk of side effects.
How to manage the interaction:
Although there is a possible interaction between Uprise D3 Drops 15 ml and calcifediol, you can take these medicines together if prescribed by your doctor. If you notice any of these symptoms - irregular heartbeat, seizures, weakness, tiredness, headache, dizziness, ringing in the ears, loss of appetite, feeling sick, dry mouth, strange taste in your mouth, muscle or bone pain, thirst, losing weight, eye infection, sensitivity to light, runny nose or itching - contact a doctor right away. Do not discontinue any medications without consulting a doctor.
Cholecalciferol and doxercalciferol are forms of vitamin D, and taking too much vitamin D may lead to toxic effects.
How to manage the interaction:
Although there is a possible interaction between Uprise D3 Drops 15 ml and doxercalciferol, you can take these medicines together if prescribed by your doctor. If you notice any of these symptoms - irregular heartbeat, seizures, weakness, tiredness, headache, dizziness, ringing in the ears, loss of appetite, feeling sick, dry mouth, strange taste in your mouth, muscle or bone pain, thirst, losing weight, eye infection, sensitivity to light, runny nose or itching - make sure to call a doctor right away. Do not discontinue any medications without consulting a doctor.
Co-administration of Cholecalciferol and Calcitriol are forms of vitamin D, and taking too much vitamin D may lead to toxic effects.
How to manage the interaction:
Although there is a possible interaction between Uprise D3 Drops 15 ml and calcitriol, you can take these medicines together if prescribed by your doctor. If you notice any of these symptoms - irregular heartbeat, seizures, weakness, tiredness, headache, dizziness, ringing in the ears, loss of appetite, feeling sick, dry mouth, strange taste in your mouth, muscle or bone pain, thirst, losing weight, eye infection, sensitivity to light, runny nose or itching - contact a doctor right away. Do not discontinue any medications without consulting a doctor.
The combined use of cholecalciferol and paricalcitol are forms of vitamin D, and taking too much vitamin D may lead to toxic effects.
How to manage the interaction:
Although there is a possible interaction between Uprise D3 Drops 15 ml and paricalcitol, you can take these medicines together if prescribed by your doctor. If you notice any of these symptoms - irregular heartbeat, seizures, weakness, tiredness, headache, dizziness, ringing in the ears, loss of appetite, feeling sick, dry mouth, strange taste in your mouth, muscle or bone pain, thirst, losing weight, eye infection, sensitivity to light, runny nose or itching - contact a doctor right away. Do not discontinue any medications without consulting a doctor.
The combined use of cholecalciferol and ergocalciferol are forms of vitamin D, and taking too much vitamin D may lead to toxic effects.
How to manage the interaction:
Although there is a possible interaction between Uprise D3 Drops 15 ml and ergocalciferol, you can take these medicines together if prescribed by your doctor. If you notice any of these symptoms - irregular heartbeat, seizures, weakness, tiredness, headache, dizziness, ringing in the ears, loss of appetite, feeling sick, dry mouth, strange taste in your mouth, muscle or bone pain, thirst, losing weight, eye infection, sensitivity to light, runny nose or itching - contact a doctor right away. Do not discontinue any medications without consulting a doctor.
Taking Cholecalciferol together with Sucralfate may increase the risk or severity of kidney problems.
How to manage the interaction:
There may be a possibility of interaction between Cholecalciferol and Sucralfate, but it can be taken if prescribed by a doctor. Do not discontinue any medications without consulting a doctor.
Cholecalciferol and dihydrotachysterol are forms of vitamin D, and taking too much vitamin D may lead to toxic effects.
How to manage the interaction:
Although there is a possible interaction between Uprise D3 Drops 15 ml and dihydrotachysterol, you can take these medicines together if prescribed by your doctor. If you notice any of these symptoms - irregular heartbeat, seizures, weakness, tiredness, headache, dizziness, ringing in the ears, loss of appetite, feeling sick, dry mouth, strange taste in your mouth, muscle or bone pain, thirst, losing weight, eye infection, sensitivity to light, runny nose or itching - contact a doctor right away. Do not discontinue any medications without consulting a doctor.
Drug-Drug Interactions Checker List
- CHOLESTYRAMINE
- CARBAMAZEPINE
- PHENOBARBITAL
- DOXYCYCLINE
- NEOMYCIN
- CHLORAMPHENICOL
- ALENDRONATE SODIUM
- LEVOTHYROXINE
- HYDROCHLOROTHIAZIDE
- DIGOXIN
Diet & Lifestyle Advise
- Include dairy products like milk, yogurt, cheese or milk-based custard in your diet.
- Eat daily a serving of broccoli, cabbage, spinach and other green leafy vegetables.
- Include the best dietary sources of vitamin D, such as fish liver oils and vitamin D–fortified milk.
- Snack on calcium-rich nuts like Brazil nuts or almonds.
- Sprinkle sesame seeds over your food, vegetables and salads. Sesame seeds are high in calcium.
- Avoid or reduce the intake of caffeine, soft drinks and alcohol that inhibit calcium absorption.
- Replace the meat with tofu or tempeh for extra calcium in your food.
Habit Forming
Side Effects of Uprise D3 Drops
- Constipation
- Increased blood calcium levels
- Increased calcium levels in urine
- Vomiting
- Nausea
- Chest pain, feeling short of breath
Special Advise
- Uprise D3 Drops may interfere with cholesterol tests, hence please inform your physician and laboratory staff that you are taking Uprise D3 Drops before undergoing blood tests.
- Clinical monitoring of serum electrolyte concentrations and cardiac function is recommended.
Disease/Condition Glossary
Vitamin D deficiency: When a person cannot get enough Vitamin D through food and exposure to sunlight, it leads to vitamin D deficiency. It often leads to thin, brittle, or misshapen bones.
Osteoporosis: It is a bone disease that weakens and brittle bones by decreasing bone density.
Tetany: A disease condition due to low levels of calcium (hypocalcemia) in the body that causes cramps and spasms in the hands, feet, and larynx (voice box).
Osteomalacia/Rickets: A disease caused by softening and weakening bones in children due to inadequate vitamin D.
Hypoparathyroidism: It is a disease characterized by low parathyroid hormone levels. This can cause low calcium levels and trigger tetany (muscle cramps, spasms, or tremors).
All Substitutes & Brand Comparisons
RX
Nutrid Drops 30 ml
Azveston Healthcare Pvt Ltd
₹76.5
(₹2.3/ 1ml)
65% CHEAPERRX
Triple-D3 Drops 30 ml
Terra Pharma Pvt Ltd
₹76.5
(₹2.3/ 1ml)
65% CHEAPERRX
Wellbaby 400IU Oral Drops 15 ml
Meyer Organics Pvt Ltd
₹41
(₹2.46/ 1ml)
62% CHEAPER

Have a query?